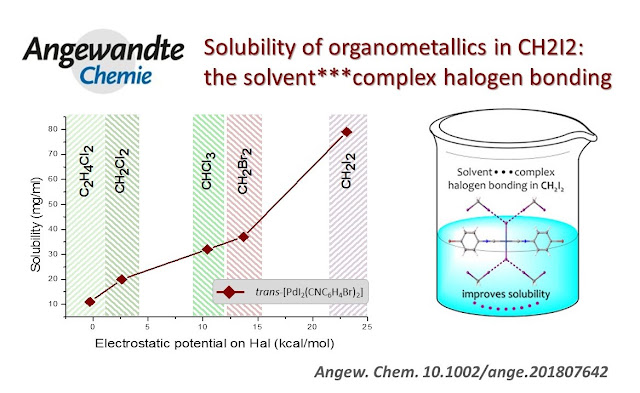
Cl/Br/I Triple Halogen Isostructural Exchange
Isomorphous Series of PdII-Containing Halogen Bond Donors Exhibiting Cl/Br/I Triple Halogen Isostructural Exchange
x
Two isomorphous series
including the adducts trans-[PdI2(CNC6H4-4-X)2]•2I2
[(1–3)•2I2; X
= Cl 1, Br 2, I 3] and the complexes cis-[PdCl2(CNC6H4-4-X)(PPh3)]
(4–6; X = Cl 4, Br 5, I 6) were
characterized by X-ray crystallography. Inspection of the XRD data allowed the
identification of halogen bonding C–X•••I–I in (1–3)•2I2 and
C–X•••Cl–[Pd] in 4–6 thus providing a rare example of the triple
Cl/Br/I exchange of s-hole donating halogens. In accord with the conducted
theoretical considerations, the strength of these XBs are in the range of 0.9–2.8 kcal/mol and these contacts are of noncovalent nature. The
calculated maximum electrostatic
potentials on the s-hole donating X centers of CNC6H4-4-X on the 0.001 a.u. molecular surfaces for both
(1–3)•2I2 and
4–6 series are increased
along with the decrease of relative XB lengths. These data are consistent with
Politzer and coworkers conclusions that are based exclusively on theoretical
ground.