Palladium(II)-Mediated Addition of Benzenediamines to Isocyanides
Palladium(II)-Mediated Addition of Benzenediamines to Isocyanides: Generation of Three Types of Diaminocarbene Ligands Depending on the Isomeric Structure of the Nucleophile

Full text: Organometallics, 2016, 35, 2, 218–228
We found that the
direction of the reaction between the isocyanide-palladium species and benzene-1,n-diamines (n =
2–4) depends
on the position of the amino group in the benzene ring, viz. on different
basicity and nucleophilicity of these nucleophiles. Benzene-1,3-diamine BDA1 reacts with only one
isocyanide group of complexes 1 and 2 leading to the typical monodentate
Pd-ADC complexes 4 and 5, respectively. The reaction between
benzene-1,4-diamine (BDA2) and complexes 1 and 2 includes
participation of both amino groups of the nucleophile accomplishing binuclear
Pd-ADC 6 and 7.
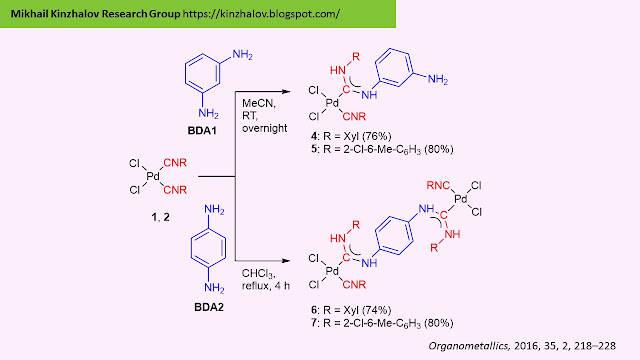 |
| Reactions of 1 and 2 with BDA1 and BDA2. |
By contrast, the
reaction of benzene-1,2-diamines BDA3–BDA5 with palladium-isocyanides opens up the route for preparation
of a wide range of aminocarbene species of different structure. Thus, a
reaction of equimolar amounts of 1,2-diamines BDA3–BDA5 and bis-isocyanide complexes 1–3
(a molar ratio 1:1) affords aminocarbene monocationic complexes 8–16.
Some of them (13 and 16) are able to form Chugaev-type C,C-chelated bis-carbene complexes 17
and 18 at long time exposition of
the reaction mixture. The addition of two-fold excess of BDA3 or BDA4 (2 equiv)
to isocyanide complexes 1–3 leads to C,N-chelated bis-carbene complexes 19–24.
 |
| Nucleophilic additions of 1,2-diamines BDA3–BDA5 to the isocyanides in 1–3 |
Prepared diaminocarbene complexes represent different types of aminocarbene palladium species and they might be potentially interesting as acyclic diaminocarbene-based catalysts for cross-coupling reactions. In this context, palladium complexes with monodentate aminocarbene ligands as well as with C,C- and C,N-chelated bidentate aminocacarbenes have demonstrated an outstanding catalytic efficiency in different organic transformations, including catalysts that outperform the most active phosphine- and NHC-based complexes. At the same time, application of these compounds in other catalyzed organic transformations, e.g., Buchwald–Hartwig amination, or Heck reaction is significantly less studied but worth exploring and works in this direction are underway in our group.