Diversity of Isomerization Patterns and Protolytic Forms in Aminocarbene Complexes
Diversity of Isomerization Patterns and Protolytic Forms in Aminocarbene PdII and PtII Complexes Formed upon Addition of N,N’-Diphenylguanidine to Metal-activated Isocyanides
Full text: Organometallics, 2017, 36 (21), pp 4145–4159
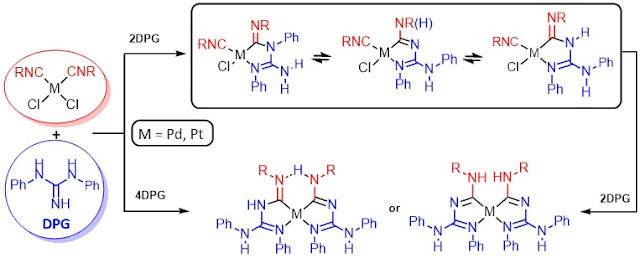
In this work we found that reaction of the palladium(II) and platinum(II) isocyanide complexes
cis-[MCl2(CNR)2]
[M = Pd, R = C6H3(2,6-Me2)
(Xyl), 2-Cl-6-MeC6H3, cyclohexyl (Cy), t-Bu, C(Me)2CH2(Me)3
(1,1,3,3-tetramethylbuth-1-yl abbreviated as tmbu); M = Pt,
R = Xyl, 2-Cl-6-MeC6H3, Cy, t-Bu, tmbu] with N,N’-diphenylguanidine
(DPG) leads to DPG-derived metal-bound
acyclic diaminocarbene (ADC) species.
This reaction occurs via the two-step
process, involving the initial coupling of the guanidine with one of the
isocyanides and leading to monocarbene monochelated species, while the next
addition grants the bis-carbene bis-chelated metal compounds; DPG
behaves as nucleophile, deprotonating base, and chelator. The addition of DPG proceeded with different regioselectivity
depending on the metal center and, in a larger extent, on the substituent R in
RNCs. The X-ray diffraction studies for the mono-
and bis-carbene complexes confirmed
the regioisomerism of these species and allowed the identification of ADC
protolytic forms stabilized in the solid-state. 1D (1H and 13C{1H}) and 2D (1H,1H-NOESY;
1H,15N-HSQC; 1H,15N-HMBC) solution NMR of the obtained systems demonstrated their
configuration isomerism accompanied with prototropic tautomerism. Together, the
solid state and solution data provide an insight into the flexible character of
ADC species.