The Role of Halogen Bonding in Solubility
The Role of Solvent···Complex Halogen Bonding in Solubility of Organometallic Species
Full text: Angew. Chem. Int. Ed., 2018, 57, 12785

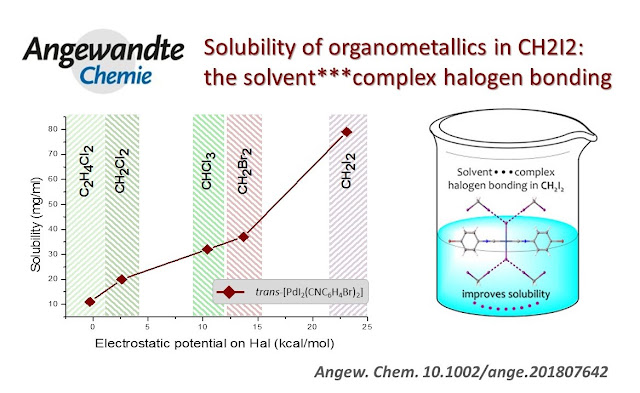
In the current study, we evaluated a solubility of a number of organometallics and showed that it is noticeably improved in diiodomethane when compared to other haloalkane solvents. Better solvation properties of CH2I2 were associated with a dramatic growth of the s-hole donating ability of this solvent, and that results in the formation of the uniquely strong solvent-(metal complex) halogen bonding. The strength of halogen bonding is attenuated by the introduction of additional halogens to the organometallic species due to the competitive formation of more favourable intermolecular complex-complex halogen bonding. Exceptional solvation properties of diiodomethane and its inertness towards organometallics make this solvent a good candidate for NMR solvent-of-choice, in particular, for the acquisition of insensitive spins.
 |
| The numbering scheme of prepared metal-isocyanide complexes used as probes for solvation properties of haloalkanes. |
Amongst
all haloalkane solvents studied, diiodomethane, CH2I2,
demonstrated the best solvation properties determined by the highest solubility
of all model compounds.
 |
Solubility vs. electrostatic potential Vs(r)max on halogen atom
in haloalkanes.
|
 |
| The solubility of palladium(II) and platinum(II) isocyanide complexes in CHCl3 and CH2I2 |
 |
| The solubility (mg/ml) of palladium(II) and platinum(II) isocyanide complexes in CHCl3 and CH2I2 depending on isocyanide ligand (CNR) and metal halide type (MHal2). |
 |
| Comparative solubility of different organometallic species in CDCl3 and CH2I2. |
 |
| The solubility (mg/ml) of studied trans square-planar non-electrolyte complexes with halogen ligands in CHCl3 and CH2I2. |
 |
The solubility (mg/ml) of studied complexes with other
geometry/configuration in CHCl3 and CH2I2.
|
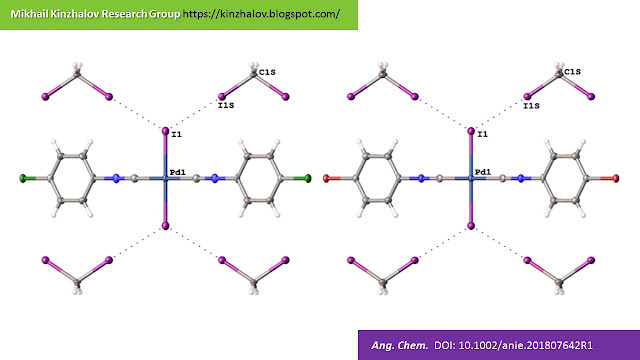 |
The C–X2···I–Pd (X2 = Br, I) XBs between two complex molecules in 11 (top) and 12 (bottom).
|
 |
| The H2C(I)–I···I–Pd XBs in 10·2CH2I2 (left) and 11·2CH2I2 (right). |
 |
| ESP distribution in 12 (X = I, Hal = I) (M06-2X/CEP-121G level of theory). Blue color – negative ESP, red color – positive ESP. |
We scrutinized the solvation properties of a number of haloalkane solvents against a series of organometallic species. Using NMR measurements, we established that CH2I2 demonstrated the best solvation properties among all haloalkanes studied; solubility values for organometallics were improved by up to an order of magnitude when compared to the commonly used CHCl3. Since no correlation between the polarity of haloalkanes and the solubility of all model complexes was observed, we looked for an alternative explanation. We anticipated that better solvation properties of CH2I2 are related to a dramatic growth of the s-hole donating ability of this solvent when compared to others, and that results in the formation of strong solvent-(metal complex) halogen bonding. The emergence of these uniquely strong XBs in diiodomethane and its inertness towards organometallics make this solvent a good candidate for NMR solvent-of-choice, in particular, for the acquisition of insensitive spins, where the concentration of an analyte is crucial.